- Mindsailors
- Markets
- Medical device design
A medical device design and development guide by Mindsailors
The medical device projects we have completed so far have helped us build long-lasting relationships with our clients and, most of all, have helped improve health and save lives for the people using them. That's why, for our designers and engineers, working on developing medical products is one of the most rewarding tasks they can take on. Fueled by this motivation, we decided to publish this medical device design and development guide.
A good medical device design takes into account compliance with healthcare regulations, solution specifications, and the needs of end users. This article is for Medtech professionals. It is a detailed guide to designing effective medical devices. You will also find here tips from our experienced design team as well as their recollections of challenges they faced when designing medical devices. Keep in mind this is not a blueprint, but if you are looking for information on the medical device design process and medical device regulations in the USA, EU, UK, Canada and Australia, you will find plenty of insightful knowledge here.
How is medical device design different from designing regular products?
Designing a medical device is fundamentally different from designing a regular consumer product due to stricter regulations, higher safety requirements, and more complex development processes. Here’s a plain explanation of the key differences:
1. Strict Regulatory Requirements
Medical devices are subject to rigorous regulations (e.g., FDA in the U.S., MDR in the EU). These regulations ensure the device is safe and effective for its intended use.
- Consumer Electronics: Must meet general safety standards (e.g., CE mark for Europe), but the bar is lower. A smartphone doesn't have to prove it saves lives; a heart monitor does.
- Medical Devices: Require extensive testing, clinical trials, and documentation before they can be sold.
2. Risk Management
Medical devices are often life-critical, meaning a failure could harm or even kill a patient. Therefore, risk management is at the core of their design.
- Consumer Electronics: Risks like a cracked screen or a short circuit are annoying but not life-threatening.
- Medical Devices: Risks must be minimized and documented through processes like ISO 14971 (risk management for medical devices).
3. Human-Centric Design with Precision
Medical devices often interface directly with the human body, so usability, ergonomics, and reliability are paramount.
- Consumer Electronics: Designed for convenience, aesthetics, and fast adoption by the general public.
- Medical Devices: Must prioritize patient safety, ease of use by clinicians, and precision. For example, a surgical tool must be ergonomic and allow precise movements.
4. Longevity and Reliability
Medical devices need to be durable and reliable across their lifecycle, as they are often used in critical conditions.
- Consumer Electronics: Products may follow a short lifecycle (a new smartphone every year).
- Medical Devices: Have long lifespans and require regular maintenance and calibration, often over decades.
5. Complex Documentation
The development of medical devices involves creating extensive documentation to comply with legal requirements.
- Consumer Electronics: Limited to user manuals and design files.
- Medical Devices: Requires design history files, risk analysis, clinical evaluation reports, and traceability matrices.
6. Interdisciplinary Collaboration
Medical devices require expertise from multiple domains: biomedical engineering, electronics, mechanical design, software, and regulatory specialists.
- Consumer Electronics: Teams often focus on market trends and features.
- Medical Devices: Teams must collaborate closely to balance performance, safety, and compliance.
7. Testing and Validation
Medical devices undergo far more rigorous testing before being approved for use.
- Consumer Electronics: Functional testing and user feedback.
- Medical Devices: Testing includes pre-clinical trials, clinical trials, and simulation of worst-case scenarios.
8. Cost of Failure
Failure in a consumer product might result in bad reviews and lost sales. Failure in a medical device can result in legal liability, recalls, and potentially loss of life.
After reading this medical device design guide you should find some time to watch our podcast episode about mistakes you should avoid when designing a medical product.
What you should first understand about medical device design
In its core, designing a medical device is a lot like any other device design process - you need to deeply understand the problem you are solving and then you need to do it better than your competitors. In itself that is already a challenge many business owners underestimate. Add to that the real and perceived importance of healthcare to people’s lives, and the significantly more complex regulations your medical device will need to meet, and you will begin to understand why medical device design is indeed a lot more difficult than designing any other type of product.
There is no space for small imperfections, free interpretation or any sort of leeway in neither any step of designing a medical device nor in introducing it to the market. Your medical device will be responsible for someone’s health, or even their life, so saying “no room for error” is saying too little.
Everything from initial research on the target user and the problem you are solving, up to the disposal of your device when it’s lifecycle comes to an end, needs to be precisely addressed. You need legal experience for patent and regulatory solutions. You need testing and certification experience for making sure you did absolutely all you could to make sure your product is safe to use, does what it's supposed to do, and can withstand all scrutiny. Of course you need solid business research and planning to make sure this tremendous effort will make a good profit in the end, and at the base of it all you need impeccable design and development experts, which are the core piece of this entire puzzle.
Did I get your attention? Now you know what you’re dealing with.
How to design a medical device?
A lot of work goes into finding the right healthcare solution that meets customer needs. A good concept requires everyone to be on the same page, have a clear definition of the scope based on what the end user needs, work together as a team, and stick to the specifications and requirements taken from the product definition at the same time minimizing risks and keeping the best quality. Seems easy? It’s not! This data gathering and decision making chain needs an experienced and rock solid link at each step.
If you are a Medtech professional looking for a way to improve the development process in your next medical device design project, then this guide is a solid foundation for you to minimize risk and maximize effectiveness.

Product life cycle stages
In this guide I will cover:
This will sound cheesy, but … let’s start from the beginning!
Medical device ideation and classification
Like in other industries, Medtech innovation starts with analyzing the market and figuring out what needs aren't being met or aren't being met well enough, or if there is a better way to meet those needs. These needs could be anything that helps solve a problem, like a new or better way to monitor health, better ways to deliver care, devices or technologies that make administration easier, or anything else that helps people stay healthy and live longer.
Usually this winds down to two categories - medical devices that alleviate an existing problem or ones that help avoid a possible problem. The former has a symptomatic effect, the latter is a prevention measure.
Where to start your ideation work?
Scratch the right itch
This might even be literal! But it doesn’t have to be. It is a good metaphor for finding a problem that people might pay good money for someone to solve. If you try to solve a “mild inconvenience”, people won’t queue in the waiting list to buy your freshly developed medical device. A “mild inconvenience” can wait. Some even learn to live with it!
But an itch that doesn’t go away? Now that’s a problem to solve!
Classifying a problem, especially a medical one, as a “mild inconvenience” or an “annoying itch” is difficult, because it’s subjective. For you it might be an inconvenience, but I might not be able to stand it. That’s why there’s more to ideation than just finding ideas.
Top 3 priorities for the medical device development conceptual design phase:
- Make sure you verify what your clients need with the clients themselves. Never assume based on personal experience or hearsay. Poll large samples and verify from every angle. If you get the problem wrong, you risk wasting a ton of money and a couple of years of work (It was shown in studies that on average it takes from three up to seven years to bring a medical device from its concept to getting it approved to hit the market);
- Medical device classification - you need to comply with medical device classification rules, which are different for different parts of the world. Our company's focus is on the regions of the USA, EU, UK, Australia, and Canada, and the regulations are different in each of them;
- Intellectual property - there is a big chance that if you have found an itch big enough to scratch, someone else is on it too. Might have even been on it for a very long time now. You need to make sure you won’t infringe on someone else’s intellectual property, or worse, someone’s patented solution.
Keep in mind these are very widely defined priorities. For these steps you need a team with expertise in research and development, marketing, patent law and medical device regulatory systems. Usually teams developing medical devices, especially ones that are to be brought to an international market are themselves international. A single team is good for developing a medical device, but to meet international regulatory standards outside legal assistance is usually necessary.
If you are looking for medical device design and development experts you can contact our team for assistance.
However you decide to proceed, here are the three most important types of experts you need on your medical device development team:
- Healthcare experts for the medical industry's clinical and scientific know-how
- Lawyers and product development experts for regulatory affairs and intellectual property
- Designers and engineers for product design, development, prototyping and DFM
In short, you first need to have your business sorted out - with the help of medical experts you can make sure you're on the right track to profit. Next you need to make sure if your concept won't meet any legal blockades from entering your desired markets. You need to identify them as soon as possible, so that your design and engineering teams are aware of what solutions they need to come up with on top of building the product itself. If you have all those three sorted out, you’re good to move to the next stage of the medical device development process.
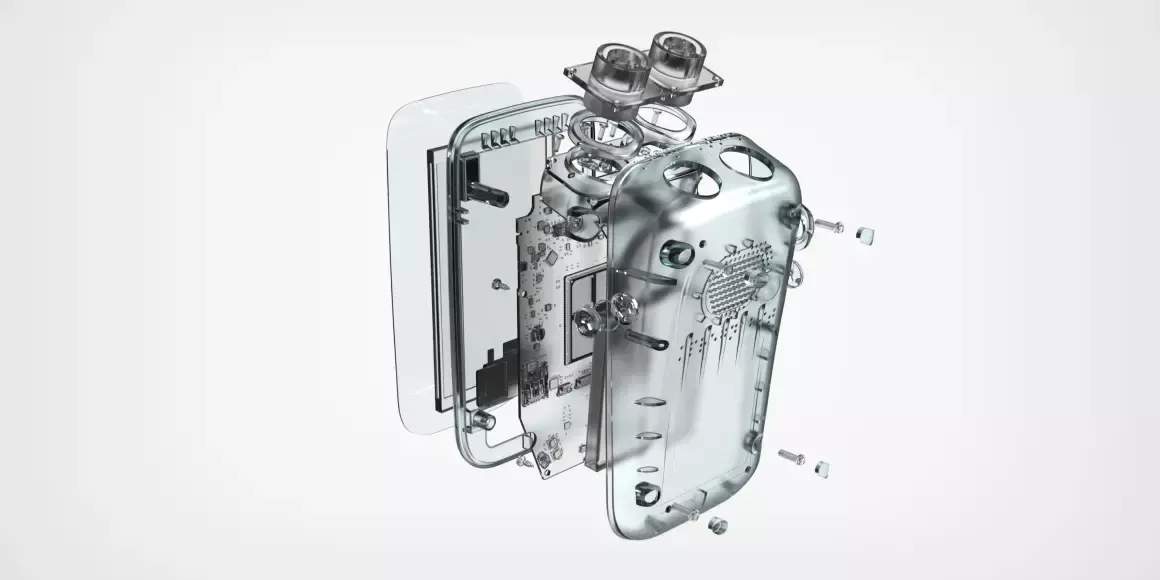
Medical device design and development
This is where your design and product development team comes into play. From delivering a proof of concept, through visual concepts, PCB design, and prototyping all the way to DFM and delivering a shelf-ready product. This is where your idea comes to life.
In itself this is a very time consuming stage. All the business and legal preparation were necessary for this stage to deliver the best result. If you won’t do your business research right, even if the design and development phase will be a success, the product will most likely fail on the market. If you won’t do your legal research right, and the development phase will deliver a great product, it might not even be allowed to enter the market.
If you want to know what might lay ahead of you, listen to this episode (Spotify link) on the IDology industrial design podcast. It’s about the most common mistakes of new product development and you can also watch the video version or read the transcription HERE.
Usually the “device design phase” is what a company focuses on first. I won’t cover it thoroughly here as it is not healthcare-specific in itself. Regulations on the other hand are, and they are the biggest hurdle for every Medtech professional aiming to develop a new medical device. So they are what I will focus on going forward.
You can learn more about our Mindsailors design process from this episode of IDology - the industrial design podcast:
Medical devices regulation
In order to get on the market, your medical device needs to meet both regional and international standards for regulatory compliance. Although they overlap, meeting international standards doesn’t mean meeting local standards at the same time. Each need to be sorted out separately. Medical device standards help define and evaluate the requirements for the design and performance of biomedical materials, tools, and equipment. They are backed by law and differ from region to region, as mentioned above.
These medical device standards let institutions in the medical device field, like product manufacturers, labs, and others, check and evaluate medical equipment and devices to make sure they are of standard quality and can be used safely and properly.
First, there are the international organizations.

The International Electrotechnical Commission (IEC)
The International Electrotechnical Commission is an international standards body that is responsible for the preparation and publication of international standards for all electrical, electronic, and associated technologies, which are generally referred to as "electrotechnology."
This is not strictly a medical matter as you probably guessed, nevertheless every device using electronics must comply with these standards.
IEC 60601-1 was the first standard of its kind for medical devices. It was written by the International Electrotechnical Commission (IEC) in 1970. IEC 60601-1, Medical electrical equipment – Part 1, is an internationally recognized standard that talks about general requirements for electrical medical equipment and devices. It covers standards for basic safety and basic performance.
Over the years, the IEC 60601-1 has gone through many changes so that it can keep up with new medical technologies and adapt to them. It's important for you to know that if you're not "in the loop", you should always check for new updates. At the time of publishing this guide the most recent changes to IEC 60601-1 came out in the form of amendments in 2020 and should be valid through 2024. You can verify the IEC medical device standards HERE. This standard has requirements for essential performance, requires usability engineering evaluations and consideration of human factors, and requires the use of a formal software development life cycle process.

PEMS life cycle model according to IEC 60601-1: software development life cycle in blue, system development life cycle in green
It also lists new and changed technical requirements for electrical and mechanical hazards, as well as new requirements for product labeling and documentation.
.svg.png)
International Organization of Standardization (ISO)
Since the 1970s, the International Organization for Standardization (ISO) has been making rules for medical devices. If you plan on developing a new medical device, it's important for you to understand these standards because they tell you how to make medical devices that are safe, effective, and meet regulatory requirements.
ISO 13485, which came out in 1996, was one of the first standards that the ISO made for medical devices. This standard explains what a quality management system for designing, developing, making, and taking care of medical devices needs to do.
ISO 14971 is another important standard for product designers. It came out for the first time in 2000. It gives advice on how to manage risks for medical devices and explains the process for finding, analyzing, and controlling risks throughout the lifecycle of a medical device.
ISO 62366 was published in 2015, and it is about how to make medical devices easier to use. This standard lists the best ways to make medical devices that are easy for their intended users, like patients and healthcare professionals, to use and understand.
In the medical device industry, ISO standards have a big impact on the work of product designers and industrial designers. These standards give a plan for designing and making medical devices that are safe, effective, and meet regulatory requirements. As a designer or design expert, using ISO standards in the design process can help you make sure that the device meets best practices in the industry, reduces the risk of bad things happening, and, in the end, improves the health of the patient.
And then, aside from these international standards, there are also standards that are specific to a certain region. All of these standards are based on international standards with only minor changes and restrictions. It is important for you to always verify the latest updates to each of the standards you need to follow.

The Food and Drug Administration (FDA), USA
If you want to develop a medical device for the USA market, you need to work in accordance with the FDA. The FDA, or the United States Food and Drug Administration, is a government organization in charge of protecting public health by regulating diverse products such as medical devices, pharmaceuticals, vaccinations, and food. The FDA's aim is to verify that these products are safe, effective, and labeled correctly.
The FDA medical device regulation includes supervising rules of medical device design, production, marketing, and distribution to ensure that they fulfill particular safety and effectiveness criteria. Medical devices are categorized into three groups based on their level of risk, with varying regulatory requirements for each.
Before medical devices can be marketed in the United States, they must be reviewed and approved by the FDA. The review procedure is determined by the classification of the device, with low-risk devices typically requiring the least amount of assessment and high-risk devices requiring the most extensive study.
Medical device makers must produce evidence that their device is safe and effective for its intended purpose, as well as meet other regulatory standards such as labeling, manufacturing controls, and post-market surveillance, in order to gain FDA clearance. The FDA also has the ability to inspect medical device manufacturing plants to ensure that relevant processes are followed.
In general, the FDA plays an important role in ensuring that medical devices available to consumers in the United States are safe and effective, as well as that they comply with regulatory standards to protect public health.
ISO standards are represented in the US by the American National Standards Institute (ANSI). Association for the Advancement of Medical Instrumentation (AAMI) and the American Society for Quality (ASQ), both of which set standards for the US, are two more groups that handle standardization in the US.
Remember however, even if you design a device with ISO standards in mind, there is a chance that the FDA will not approve it. It is important to say that the FDA aims to line their standards with international standards, yet up to the date we publish this guide it is still an ongoing process.
The FDA has its own set of rules for managing risks, which are based on both international and local standards, these rules include:
ISO 14971:2007, Medical devices – Risk management for medical devices (international standard.)
ANSI/AAMI/ISO 14971:2007 (R2010), Medical devices – Risk management for medical devices (A regional standard with additions and modifications from the referred international standard.)
When it comes to the ISO 13485 standard for quality management, it does not follow either the international or regional version. This is because the FDA has different rules for how medical devices for the US market should handle quality management.

The European Committee for Standardization and the European Committee for Electrotechnical Standardization, the European Union
If you plan for your new medical device to be sold in the EU, you will need to play by the rules of CEN and CENELEC. CEN works closely with the European Union (EU) and its member states to develop harmonized standards for medical devices that comply with EU regulatory requirements. These standards are used by manufacturers to demonstrate compliance with essential requirements under the EU Medical Devices Regulation (MDR) and In Vitro Diagnostic Medical Devices Regulation (IVDR) before placing their products on the EU market.
In addition to developing standards, CEN also provides training, certification, and other services related to medical devices to support compliance with EU regulatory requirements.
The main difference between CEN and CENELEC is the type of products they regulate. CEN develops standards for products that are not related to electrical or electronic components, while CENELEC develops standards for electrical and electronic products. However, both organizations work closely together and with the European Union (EU) to develop harmonized standards that ensure the safety, effectiveness, and compliance of products, including medical devices, in the EU market.
For the European Union, the European Committee for Standardization (CEN) is the standard that was taken from ISO, and the European Committee for Electrotechnical Standardization (CENELEC) is the regional standard that was taken from IEC. Both handle EU medical device regulation.
CEN regulations have been modified a bit to meet ISO's needs and are written with a prefix. For e.g.:
EN ISO 13485:2016, Medical devices — Quality management systems — Requirements for regulatory purposes
EN ISO 14971:2021, Medical devices — Risk management for medical devices
The EU sets these standards but does not publish them officially, and each member country has to publish them locally in their local language, hence each country then adds its own prefix to them. For example the prefix BS-EN-ISO denotes a standard that has been released in British English by the British Standards Institute.

The Canadian Medical Devices Directorate, Canada
The MDD is a division of Health Canada, Canada's federal department in charge of public health. If you plan to design a medical device and manufacture or sell it in Canada, you will need to abide by their regulations as the MDD is in charge of medical device regulation in Canada and ensuring that specific safety and effectiveness standards are met.
The Canadian Medical Devices Regulations explain the MDD's regulatory standards for medical devices. These regulations provide a risk-based classification system for medical devices, similar to that used by the FDA in the US and by organizations in the European Union.
Before marketing medical devices in Canada, manufacturers must get a license from the MDD. The MDD reviews applications for medical device licenses and evaluates the devices' safety and effectiveness based on their risk classification. High-risk medical devices may necessitate more thorough testing and clinical data before they can be sold in Canada.

The Medicines and Healthcare products Regulatory Agency, The United Kingdom
The MHRA in the United Kingdom works together with other national and international regulatory agencies to establish and standardize medical device standards. If you plan to market your medical device in the UK, this should be your focus.
Organizations such as the International Organization for Standardization (ISO), the European Committee for Standardization (CEN), and the European Committee for Electrotechnical Standardization (CENELEC) develop standards that are widely recognized and used by the medical device industry to ensure regulatory compliance. The MHRA refers to these standards for creating and enforcing its own medical device regulatory criteria. When appropriate, the agency may adopt components of these standards into its own regulatory framework to guarantee that medical devices put on the UK market fulfill certain quality and safety criteria.
The MHRA, for example, may require medical device manufacturers to demonstrate conformity with certain ISO or CEN standards as part of their marketing permission application. In some situations, the MHRA may collaborate with other regulatory bodies to set or amend these standards in order to reflect evolving technologies or emerging public health threats.

The Therapeutic Goods Administration, Australia
The TGA, a branch of the Department of Health in Australia, is in charge of medical device regulation. Before medical devices can be promoted or sold in Australia, the TGA must review their safety, quality, and performance.
To bring a novel medical device to the Australian market, the producer must file an application with the Australian Registry of Therapeutic Products (ARTG). The application must include thorough device information, such as intended usage, specs, and performance data. The TGA evaluates the application to ensure that the device meets the necessary safety, quality, and effectiveness standards.
Australia's medical device regulatory criteria are based on a risk-based approach, similar to those used in other nations such as the United States and the European Union. The TGA categorizes medical devices into four categories depending on their danger to public health, with higher-risk devices necessitating more stringent testing and review.
The TGA not only evaluates applications for new medical devices, but it also monitors the safety and performance of medical devices that are currently on the market. The agency has the authority to recall or remove devices that endanger public health, as well as to demand manufacturers to give more information or data to support the safety and effectiveness of their products.
FDA’s Rules for Design Control (USA)
The FDA design controls that also apply to medical devices are a set of guidelines that govern the design and development of medical devices in the United States. Medical device manufacturers must adhere to a defined design process that involves particular stages and methods for designing, manufacturing, testing, and validating medical devices, according to the laws. The regulations' goal is to ensure that medical devices are safe and effective for the intended usage.
Medical device makers are required by the FDA's design control standards to document and validate every step of the design process, from initial concept to final product. This includes keeping detailed records, making rigorous risk assessments, and testing and verification activities at various stages of the design process. Manufacturers must also define and adhere to protocols for managing design modifications, correcting design flaws, and guaranteeing continuing regulatory compliance.
Many other countries have similar legislation and design control requirements, including the European Union, Japan, Canada, and Australia. These regulations are frequently based on international standards, such as ISO 13485:2016, which I mentioned above.
Also, the FDA includes requirements for Current Good Manufacturing Practice (CGMP).
The FDA established the Current Good Manufacturing Practices (CGMPs) standards, which govern the manufacturing, testing, and control of medical devices in the United States. The cGMP regulations are intended to ensure that medical devices are made consistently to fulfill precise quality standards and are safe and effective for their intended application.
All phases of medical device manufacturing are covered by the cGMP rules, including design, development, production, testing, packaging, labeling, storage, and distribution. Manufacturers are required to design and maintain a complete quality system that includes written procedures, record-keeping, and constant monitoring and analysis of manufacturing processes.

Schema of FDAs risk management process for developing medical devices
Medical device makers are mandated under cGMP rules to:
- Create and keep a quality management system in place that includes procedures for managing and monitoring all phases of device manufacture
- All manufacturing activities, including design and development, raw material and component sourcing, production, testing, and post-market surveillance, must be documented and kept on file
- Develop and maintain nonconforming product handling and control procedures, including procedures for investigating and correcting any violations from established quality standards
- Continuously monitor and analyze manufacturing processes in order to detect and correct any quality concerns or discrepancies
- Develop and manage a system for maintaining and upgrading device specifications and procedures, including change control and manufacturing process validation procedures
The cGMP rules are critical to assuring the quality and safety of medical devices in the United States. The regulations ensure that medical devices are consistently manufactured to fulfill specified quality standards and that they are safe and effective for their intended use by defining clear and rigorous quality system requirements.
The Control Process for Medical Device Design, FDA, USA
The FDA defines the steps of medical device design control in the United States as follows:
- The first step comprises planning and defining the scope of the project, which includes the intended application of the device, user needs, and regulatory criteria that must be addressed. Establishing design inputs and developing a design plan are also part of this stage.
- Design and development planning involves planning and defining the scope of the project, including the intended use of the device, the user requirements, and the regulatory requirements that must be met. This stage also includes establishing design inputs and creating a design plan. The key elements of this stage are to establish a design and development plan, define the design inputs, and develop the design output.
- Design Inputs: The third stage entails obtaining and specifying design inputs, which may include safety, performance, durability, and other device aspects.
- Design Outputs: The fourth step is creating and documenting design outputs, which are the end outcomes of the design process. Specifications, drawings, and other documentation that define the device's design and intended usage are examples of outputs.
- Design Review: In the fifth stage, the design outputs are reviewed to ensure that they meet the design inputs as well as the regulatory requirements.
- Design Verification: The sixth stage entails testing, analyzing, and other ways to ensure that the design meets the design inputs as well as the regulatory requirements.
- Design Validation: The seventh step entails verifying the design through testing and other means to ensure that it satisfies the demands of the user and is appropriate for the intended usage.
- Stage number eight involves translating the design to production, which includes drafting manufacturing instructions and validating the manufacturing process.
- Design Changes: The ninth stage entails managing design changes through a rigorous change control procedure, which includes examining the impact of modifications on the device's safety and effectiveness.
- Design History file - the final stage involves creating and maintaining a Design History File (DHF) for the device. This file should contain all records pertaining to the design and development of the device, including design inputs, design outputs, verification and validation records, design review records, and design changes. The purpose of the DHF is to provide a complete record of the device's design history, which can be used to demonstrate compliance with the FDA's design control requirements. The key elements of this stage are to create and maintain the DHF, which serves as a complete record of the device's design history.

Medical device design control waterfall process
Similar rules apply in all markets, but are often a part of already existing standards like the ISO, QMS.
Testing medical devices
Medical device design and development testing in the EU, UK, Canada and Australia
Specific requirements exist in the EU, UK, Canada, and Australia for testing medical devices during the design and development process to ensure their safety and effectiveness.
Medical devices in the EU are governed by the Medical Devices Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR). (IVDR). The MDR and IVDR require manufacturers to conduct testing and evaluation in order to demonstrate regulatory compliance and ensure the safety and effectiveness of their devices. Specific testing requirements vary depending on the device classification and intended use, but generally adhere to the FDA's testing procedures.
The Medicines and Healthcare products Regulatory Agency regulates medical devices in the United Kingdom. To ensure the safety and effectiveness of medical devices, the MHRA requires manufacturers to adhere to the same standards and regulations as the EU, including the MDR and IVDR.
Medical devices in Canada are governed by the Medical Devices Regulations (MDR), which require manufacturers to conduct testing and evaluation to ensure the safety and effectiveness of their products. Specific testing requirements vary depending on the device classification and intended use, but generally adhere to the FDA's testing procedures.
The Therapeutic Goods Administration regulates medical devices in Australia. (TGA). Manufacturers are required by the TGA to conduct testing and evaluation to ensure the safety and effectiveness of their devices, as well as to comply with the Australian Regulatory Guidelines for Medical Devices (ARGMD). Specific testing requirements vary depending on the device classification and intended use, but generally adhere to the FDA's testing procedures.
In the EU, the MDR and IVDR provide guidance on the testing requirements for medical devices. The regulations require that devices undergo a conformity assessment process that includes testing and evaluation to demonstrate compliance with the regulations and to ensure the safety and effectiveness of the device. The specific tests required will depend on the classification of the device and its intended use.
Similarly, in the UK, Canada, and Australia, regulatory agencies provide guidance on the testing requirements for medical devices. The MHRA in the UK, Health Canada in Canada, and the TGA in Australia have their own regulations and guidelines for testing medical devices, which are designed to ensure the safety and effectiveness of the devices.
Medical device design and development testing in the USA
In the US, to ensure the safety and effectiveness of their devices, the FDA requires medical device manufacturers to conduct testing and evaluation throughout the design and development process. Specific testing procedures vary according to the type of device and its intended use, but generally include the following steps:
- Design verification entails testing the device design to ensure that it conforms to the design inputs and specifications. This may include performance, durability, and reliability testing.
- Design validation entails testing the device in a simulated or real-world environment to ensure that it performs as expected and meets the user requirements. Testing for safety, efficacy, and usability may be included.
- Biocompatibility testing entails ensuring that the device is biocompatible and does not cause harmful reactions when used in the body. This may include cytotoxicity, sensitization, and irritation testing.
- Sterilization validation entails testing the device to ensure that the sterilization process used to prepare it for use is effective and does not jeopardize the safety or effectiveness of the device.
- Software validation entails testing any device's software components to ensure that they function as intended and do not introduce errors or bugs.
- Clinical trials: The FDA may require clinical trials for certain types of devices to evaluate the device's safety and effectiveness in actual use.
In the US, the FDA provides guidance on the testing requirements for medical devices. The FDA requires that testing be conducted in accordance with Good Laboratory Practice (GLP) regulations, which are designed to ensure the quality and integrity of non-clinical laboratory studies. The specific tests required will depend on the classification of the device, but may include bench testing, animal testing, and clinical trials.
Medical device testing summary
In all regions, the tests themselves are regulated to ensure that they are conducted in accordance with established standards and guidelines. For example, the FDA regulates the use of GLP regulations in non-clinical laboratory studies, and the EU requires that tests be conducted in accordance with appropriate standards and guidelines, such as ISO 10993 for biological evaluation of medical devices. Similarly, regulatory agencies in other regions have their own guidelines and standards for testing medical devices.
In summary, companies must decide on how to test their medical device in development based on the regulations and guidelines in the region where the device will be marketed. The specific tests required will depend on the classification of the device and its intended use, and the tests themselves are regulated to ensure that they are conducted in accordance with established standards and guidelines.
Methods for handling risks
ISO 14971:2019 is a global standard that describes a risk management process for medical devices. Compliance with this standard is required for regulatory approval in many countries, including the United States, the European Union, the United Kingdom, Canada, and Australia.
Medical device manufacturers must follow a risk management process that includes the following steps in order to comply with ISO 14971:2019:
- Risk assessment entails identifying potential hazards associated with the device and estimating the severity and likelihood of harm occurring
- Risk evaluation: Determine whether the identified risks are acceptable and, if not, whether risk reduction is required
- Risk management entails implementing risk controls to reduce identified risks to an acceptable level
- Risk benefit analysis: Assess the device's overall benefits and risks
- Risk communication entails communicating the device's risks and benefits to users, patients, and other stakeholders
- Review the risk management process on a regular basis to ensure that it is still effective and up to date.
Medical device manufacturers can demonstrate that their device is safe and effective for its intended use by following this risk management process and complying with ISO 14971:2019.
Furthermore, compliance with this standard is frequently required for regulatory approval in many regions, including the United States, the European Union, the United Kingdom, Canada, and Australia.
Why is it important to have a process for managing risks?
It is critical to have a risk management process in place because it helps ensure that products and services are safe and reliable for their intended use. A risk management process enables you to identify potential hazards, assess the likelihood and severity of harm, and implement risk-mitigation or risk-elimination measures.
When you manage risks you can:
- Protect users and customers: Risk management aids in the prevention of harm to users and customers, which can result in injury, illness, or even death
- Ensure regulatory compliance: Many industries are governed by rules and regulations that necessitate risk management processes. Compliance with these requirements is required to ensure that products and services can be sold or provided legally
- Increase customer satisfaction, loyalty, and brand value: Effective risk management can improve a company's reputation for quality and dependability, leading to increased customer satisfaction, loyalty, and brand value
- Reduce costs: You can avoid costly redesigns or recalls later on by identifying and managing risks early in the design and development process
How do procedures for risk management work?
The steps of the risk management process are shown in the picture above. The first step is to find the risks, and then the risks are measured based on how dangerous the risks are and how likely they are to happen.
If the risk level found is higher than the criteria set, it needs to be dealt with. The level of risk depends on many things, such as the device, the technology, or even the company's policy on how much risk it is willing to take.
Before putting the finishing touches on a design, it is a good idea to do a hazard analysis to find out what the usual risks are. You can easily do a primary hazard analysis by looking at the main parts and requirements for operation, such as raw materials and wastes, hardware, monitoring and control systems, human-device interfaces, and services, and then figuring out what risks might be involved.
There are some risks that need to be looked at:
- The toxicity, combustibility, and reactivity of raw materials and wastes
- Sensitivity to temperature, humidity, and other environmental factors
- Dangers from machines or electronics
- Risks related to human factors, such as ineffective delivery, drug administration, wrong or incomplete information, and control of life-sustaining operations
When more than one risk is found, they can be put in order of how dangerous they are. There are often times when you don't have enough information to spot dangers. In this case, you might try to figure out what it is by looking at similar devices and their history.
During the phase of making a prototype, you need to do a thorough analysis of the risks and hazards. There are two ways to look at hazards:
- Bottom-up analysis methods that might be used are hazard and operability (HAZOP) and failure mode effects analysis (FMEA). HAZOP is great for designs that are complicated and have many steps. FMEA is great for devices with many mechanical parts, but it takes a lot of time.
- A top-down method would be the fault tree analysis, which lets you find top-level unwanted output by looking at a combination and a series of lower-level events.
Conclusion
Introducing a new medical device to the markets of the United States, the European Union, the United Kingdom, Canada, and Australia can be a complex and difficult process. Each market has its own set of medical device regulatory requirements and standards, which can vary greatly. This means that you will need to navigate multiple regulatory frameworks and compliance requirements to ensure that your device can be legally sold or provided in these markets.
To successfully market your device, you must first develop a thorough understanding of the regulatory requirements and standards for each market, and then design and develop your device accordingly. Before you can begin selling or providing your device, you will also need to conduct extensive testing and clinical trials to demonstrate its safety and effectiveness, as well as obtain regulatory approvals or clearances.
You will also need to set up a quality management system to ensure that your device is manufactured and distributed in accordance with regulatory requirements, as well as maintain ongoing compliance with evolving regulatory standards and requirements.
I hope this helps you understand why developing a medical device on the average takes from three up to seven years. This plethora of processes with experts attached left and right shows just how much time, resources, know-how and determination one must have to pull it through.
So yes, launching a new medical device into multiple markets is a major undertaking that necessitates extensive knowledge, resources, and expertise in regulatory affairs, quality management, and product development. It can, however, be a highly rewarding opportunity to improve patient outcomes and advance healthcare innovation with careful planning and execution.
If you feel like you need to find expert partners to join your team, you can contact our medical device design experts to talk about your next project. Any path you choose, we wish you the best of luck, determination and of course a great success!
Before contacting an industrial design studio of your choice, you might want to go through this IDologu episode about preparing a project brief that will get you the best results.
Medical device design and development
Schedule an initial talk and get to know us better! You already have a basic brief? Send it over so we can have a more productive first meeting!
a meeting

